New Wave of Cancer Treatments Could Change the Game for Patients - and Investors
Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – The rising burden of cancer is poised to strain both healthcare systems and patient finances, especially as cases continue to climb. At the same time, signals out of Washington suggest a potential 40% cut to National Cancer Institute (NCI) funding, raising questions about how much support will be available from public institutions. Bloomberg has also highlighted growing alarm over the affordability of cancer therapies, with drug prices outpacing access. Against this backdrop, investors are increasingly turning their attention to emerging biotechs pursuing innovative approaches — including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Exelixis, Inc. (NASDAQ: EXEL), ORIC Pharmaceuticals, Inc. (NASDAQ: ORIC), Y-mAbs Therapeutics, Inc. (NASDAQ: YMAB), and Nurix Therapeutics, Inc. (NASDAQ: NRIX).
Forecasts for the global oncology drug market continue to climb, with Nova One Advisor projecting it will reach US$366.24 billion by 2034, reflecting a steady 7.4% CAGR. Other firms are even more bullish: ResearchAndMarkets sees the sector hitting US$866.1 billion, while Vision Research Reports puts the figure at over US$903.81 billion by the same year — citing rising demand for next-gen diagnostics and targeted therapies. For investors, the data suggests a window of opportunity is opening for emerging cancer stocks poised to scale.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) has entered a new chapter with the appointment of Jared Kelly as Chief Executive Officer and member of the Board. With a background in high-value biotech transactions and late-stage development strategy, Kelly brings experience that may help position the company for its next phase of clinical and corporate progress.
Prior to joining Oncolytics, Kelly was General Counsel at Ambrx Biopharma, where he played a key role in the company’s $2 billion acquisition by Johnson & Johnson. He also advised a range of life sciences firms on partnerships, licensing, and M&A during his tenure at Kirkland & Ellis LLP and Lowenstein Sandler LLP. His arrival comes as Oncolytics continues advancing pelareorep, a viral-based immunotherapy being evaluated in combination with checkpoint inhibitors and other agents across multiple cancer indications.
“Pelareorep’s clinical data across multiple tumors is striking and represents the potential for a true backbone immunotherapy to address many in-need indications. Importantly, the data show that pelareorep creates a robust immunologic response in difficult tumors and increases survival in a patient population where survival has historically evaded most patients,” said Jared Kelly, CEO of Oncolytics Biotech. “With a renewed focus and sharpened clinical development plan, we believe we will move pelareorep forward effectively and efficiently to a place where potential partners will see the value of a de-risked immunotherapy. I am excited to get to work accelerating development and unlocking significant value for stakeholders.”
Kelly’s appointment appears aligned with a focused strategy: advancing pelareorep through late-stage development while maintaining capital efficiency and openness to potential partnerships. The company’s lead program continues to generate data that support further investigation across several difficult-to-treat cancers.
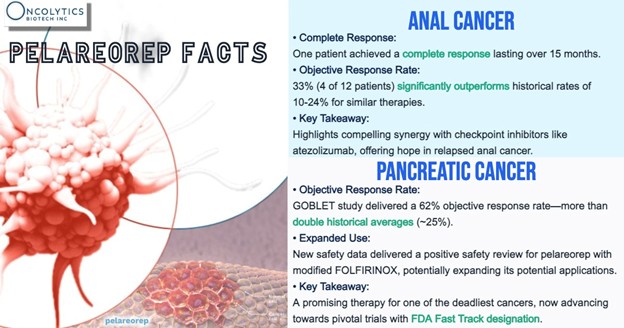
Pelareorep already holds FDA Fast Track designation in two separate indications — metastatic pancreatic ductal adenocarcinoma (mPDAC) and HR+/HER2- metastatic breast cancer (mBC) — a distinction that highlights regulatory interest in its potential.
Across clinical studies, the viral-based immunotherapy has consistently shown signs of immune activation, combinability with checkpoint inhibitors and chemotherapy, and efficacy in heavily pretreated populations.
In mPDAC, a Phase 2 cohort from the trial has reported objective response rates (ORR) above 60% in tumor-evaluable patients, exceeding historical benchmarks for this indication. Additional analyses have noted extended two-year survival rates compared to previous benchmarks. In HR+/HER2- mBC, two randomized Phase 2 trials (IND-213 and BRACELET-1) observed overall survival trends that support continued clinical evaluation.
Elsewhere, a Phase 2 anal cancer cohort combining pelareorep with a checkpoint inhibitor demonstrated partial or complete response rates that exceeded historical control trials for checkpoint inhibitor monotherapy, suggesting potential utility beyond the company’s lead programs.
“Mr. Kelly’s vision and track record is an extraordinary fit with the standout clinical data pelareorep has generated to date,” said Wayne Pisano, Chair of the Board and outgoing Interim CEO of Oncolytics. “We believe Mr. Kelly’s well-documented ability to prioritize clinical program development, execute successful financings, and attract the attention of large industry peers will help maximize Oncolytics’ potential to deliver transformative outcomes for patients and exceptional value for investors.”
Kelly’s compensation framework includes equity-based awards and performance-linked incentives tied to future financings and strategic outcomes. The structure is designed to align leadership priorities with long-term shareholder value while reinforcing a disciplined approach to capital and partnership development.
As multiple cohorts advance within the GOBLET study — including those in pancreatic and anal cancers backed by external funding and regulatory support — Oncolytics appears positioned to continue its progress with a blend of clinical momentum, financial flexibility, and sharpened strategic direction.
Prior to Kelly’s appointment, Oncolytics presented new data from its GOBLET trial at the 2025 ASCO Annual Meeting, highlighting pelareorep’s ability to stimulate both innate and adaptive immune responses in metastatic pancreatic cancer. With fresh clinical insights and new leadership in place, the company appears positioned to advance both its scientific and strategic priorities in tandem.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Exelixis, Inc. (NASDAQ: EXEL) recently reported positive topline results from its Phase 3 STELLAR-303 trial, showing that zanzalintinib combined with atezolizumab (Tecentriq) significantly improved overall survival in patients with previously treated metastatic colorectal cancer.
The trial met one of its dual primary endpoints, demonstrating a survival benefit over regorafenib in the full intent-to-treat population.
"The STELLAR-303 results, which showed a survival benefit with the combination of zanzalintinib and atezolizumab versus regorafenib across all randomized patients with previously treated metastatic colorectal cancer, marks an important first milestone for our zanzalintinib pivotal development program," said Amy Peterson, M.D., Executive Vice President, Product Development & Medical Affairs, and Chief Medical Officer, Exelixis. "We look forward to discussing the findings with regulatory authorities and presenting the detailed results at an upcoming medical conference."
Safety outcomes were consistent with prior observations, and the company plans to share full data at an upcoming medical conference.
ORIC Pharmaceuticals, Inc. (NASDAQ: ORIC) has announced promising preliminary data from its ongoing Phase 1b study of ORIC-944 combined with approved androgen receptor (AR) inhibitors in patients with metastatic castration-resistant prostate cancer (mCRPC). The treatment showed 59% of patients achieved a PSA50 response rate among 17 evaluable patients.
“The data generated to date continue to demonstrate the potential of ORIC-944 to be a best-in-class PRC2 inhibitor that may benefit a broad range of patients with prostate cancer,” said Jacob M. Chacko, M.D., President and CEO of ORIC. “The efficacy and safety data presented today compare favorably to other PRC2 inhibitor data presented earlier this year. We look forward to subsequent updates from the dose exploration and dose optimization portion of the Phase 1b trial over the next three quarters as we move towards initiating our first global registrational trial in 1H 2026.”
ORIC-944 also demonstrated a favorable safety profile, with no dose-limiting toxicities or serious unexpected events observed. These early results support continued development and potential best-in-class positioning in the mCRPC landscape.
Y-mAbs Therapeutics, Inc. (NASDAQ: YMAB) recently provided a clinical and pipeline update on its radiopharmaceutical R&D programs during a virtual investor event. The company emphasized progress on its lead asset, 177Lu-DOTA, including Trial 1001 which is in adult and adolescent patients with recurrent or refractory metastatic solid tumors, including small cell lung cancer, sarcomas, malignant melanomas, and high-risk neuroblastoma. Additionally, Y-mAbs has selected lung cancer, women’s cancers, and gastrointestinal cancers as target oncology franchise-expanding opportunities.
“At Y-mAbs, our mission is to deliver innovative therapeutic solutions for life-threatening diseases and improve the lives of patients and their families,” said Michael Rossi, President and CEO of Y-mAbs. “We are excited to provide these updates across our Radiopharmaceutical Business today, share data confirming our pretargeted approach has been validated in humans, and reiterate the potential of our platform to deliver novel products that we believe will have a meaningful impact on how we treat certain cancers.”
Nurix Therapeutics, Inc. (NASDAQ: NRIX) recently presented new clinical data showing that its lead BTK degrader bexobrutideg (NX-5948) continues to deliver durable and increasingly deep responses in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and Waldenström macroglobulinemia. Among heavily pretreated CLL patients, an 80.9% objective response rate (ORR) was recorded and one patient who achieved a complete response.
“With longer time on treatment, we are encouraged by the continued favorable safety profile and high ORR with deepening responses in CLL and WM, including those with clinical and genomic high risk features including CNS involvement,” said Paula G. O’Connor, M.D., chief medical officer of Nurix. “We have further expanded our Phase 1b cohorts in select CLL populations and remain on track to initiate pivotal studies in 2025.”
The therapy was also well-tolerated, showing a manageable safety profile across doses.
CONTACT:
Baystreet.ca
(250) 999-4849
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.