Investor Attention Turns to Biotech as Traditional Cancer Research Models Strain
Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – As traditional research institutions face growing budget constraints, the burden of advancing cancer treatment is shifting more heavily onto the shoulders of private enterprises. Early onset cancer diagnoses are rising, and with federal funding at NIH under pressure, the most promising innovations may increasingly come from nimble, precision-driven biotech firms. Among those gaining attention from investors are Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), NovoCure Limited (NASDAQ: NVCR), Nuvation Bio Inc. (NYSE: NUVB), Guardant Health, Inc. (NASDAQ: GH), and SELLAS Life Sciences Group, Inc. (NASDAQ: SLS).
Analysts are forecasting a powerful tailwind for oncology markets. According to Precedence Research, immunotherapy alone is projected to reach a global market value of US$1.2 trillion by 2033, growing at an annual rate of 18%, while Vision Research Reports predicts broader oncology spending to top US$900 billion by the end of the decade. As major treatment modalities evolve—from cell therapy to real-time diagnostics—investors are increasingly focused on the companies pushing boundaries in clinical development, delivery, and disease monitoring.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) is gaining new visibility ahead of its upcoming presentation at the 2025 ASCO Annual Meeting, where the company will unveil new clinical trial data on pelareorep’s immunological activity in pancreatic cancer. The data, drawn from the GOBLET study, highlights how pelareorep appears to convert immunologically “cold” tumors into “hot,” inflamed environments—potentially making them more vulnerable to immune attack.
Specifically, new analyses show pelareorep induces a pro-inflammatory tumor microenvironment (TME) and activates both innate and adaptive immunity. This is a rare achievement in pancreatic ductal adenocarcinoma (PDAC), a cancer type widely considered resistant to immune-based therapies.
“For the first time, we’re able to map the cascade of immune responses stimulated by pelareorep,” said Thomas Heineman, M.D., Ph.D., Chief Medical Officer for Oncolytics. “It starts with the expansion of anti-reovirus T cells, followed by the upregulation of chemokines that mediate the expansion of pre-existing TIL (tumor-infiltrating lymphocyte) clones in the blood.”
According to Heineman, these immune cells don’t just expand in the bloodstream—they’re believed to return to the tumor itself and help shrink it.
“These T cells can now return to the tumor and attack it, resulting in a reduction in tumor size,” Heineman added. “Pelareorep-mediated upregulation of chemokines also makes the tumor microenvironment immunologically active and able to actively recruit cancer-specific T cells to the tumor. These findings deepen our understanding of pelareorep’s ability to convert immunologically cold tumors into immunologically active ones that may benefit from pelareorep-based combination therapy.”
The abstract, titled “Role of pelareorep in activating anti-tumor immunity in PDAC,” (Abstract #2562) will be presented as a poster during the Developmental Therapeutics – Immunotherapy session on June 2, 2025. A copy will be made available on the Media page of Oncolytics’ website following the session.
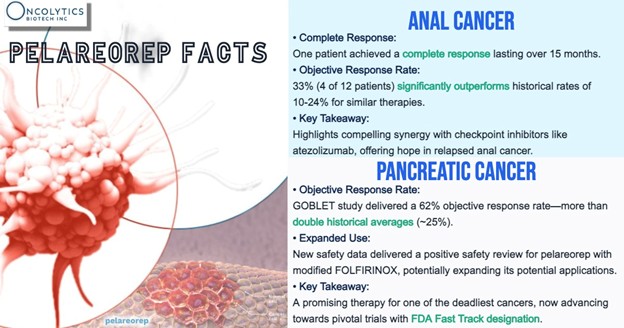
This new mechanistic insight builds on prior efficacy data from GOBLET Cohort 1, where pelareorep—combined with nab-paclitaxel, gemcitabine, and the checkpoint inhibitor atezolizumab—produced a 62% overall response rate, 85% disease control rate, and 45% 12-month survival rate in first-line metastatic PDAC patients.
For context, GOBLET is a multi-cohort, phase 1/2 study evaluating pelareorep in combination with various immunotherapy and chemotherapy regimens across gastrointestinal cancers. Conducted in partnership with AIO-Studien-gGmbH in Germany, the trial uses an adaptive design: cohorts meeting efficacy thresholds may expand enrollment. In pancreatic cancer, this trial is a proving ground for pelareorep’s use in first-line and newly diagnosed settings—potentially setting up future pivotal decisions.
Progress continues elsewhere in the GOBLET study as well. In Cohort 5, newly diagnosed metastatic PDAC patients received pelareorep with modified FOLFIRINOX, with or without atezolizumab. After completing the safety run-in in six evaluable patients, the study has been cleared to proceed by both Germany’s Paul-Ehrlich-Institut and an independent data safety monitoring board. This arm is backed by a US$5 million PanCAN grant, with further data expected in 2026. Favorable data from this cohort could expand pelareorep’s potential addressable market in this indication.
Meanwhile, in anal cancer, Cohort 4 has already reported signs of durable response. Of 12 evaluable patients treated with pelareorep and atezolizumab, four achieved partial responses, and one reached a complete response lasting more than 15 months—results that surpass historical benchmarks for checkpoint inhibitors alone. The cohort is now being expanded to validate these findings and assess registrational potential.
In breast cancer, the recently completed randomized phase 2 BRACELET-1 trial in HR+/HER2- metastatic disease showed patients receiving pelareorep plus paclitaxel nearly doubled their progression-free survival compared to paclitaxel alone. These outcomes are supportive of those seen in a prior randomized phase 2 study and strengthen the case for a pivotal trial.
Key opinion leaders continue backing pelareorep’s approach. In a recent panel hosted by H.C. Wainwright, Profs. Martine Piccart and Alexander Eggermont emphasized how pelareorep may help “turn cold tumors hot”—a key requirement for making immunotherapies effective in traditionally resistant cancers.
While still in the clinical development stage, pelareorep has demonstrated compatibility with multiple chemotherapies and checkpoint inhibitors, suggesting it could function as a plug-in immune booster across diverse treatment regimens. Its intravenous delivery, systemic impact, and favorable safety profile further support its adaptability in combination trials.
“Pelareorep continues to build clinical momentum, delivering encouraging results in challenging cancer types and has the potential to extend and improve the lives of patients,” said Wayne Pisano, Chair of Oncolytics’ Board of Directors and Interim CEO. “This versatility and broad potential applicability are achieved via intravenous administration and the ability to combine with chemotherapies and checkpoint inhibitors while maintaining a favorable safety profile.”
As it stands, Oncolytics may be entering a stretch where scientific validation, clinical optionality, and capital flexibility are all converging. The company ended Q1 2025 with $15.3 million in cash and a US$20 million equity facility from Alumni Capital, giving it financing control without restrictive terms or dilutive warrants.
With fresh data coming out of ASCO and multiple arms of GOBLET advancing, pelareorep’s immune-activating potential appears to be gaining traction across an expanding range of solid tumor indications.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
NovoCure Limited (NASDAQ: NVCR) will also be at the 2025 ASCO Annual Meeting to present full Phase 3 data from its PANOVA-3 trial, highlighting the use of Tumor Treating Fields (TTFields) therapy in combination with gemcitabine and nab-paclitaxel for first-line treatment of locally advanced pancreatic cancer. The study met its primary endpoint, showing a statistically significant improvement in median overall survival versus chemotherapy alone.
TTFields therapy, which uses electric fields to disrupt cancer cell division, has already been approved in several aggressive tumor types and is being studied across multiple additional indications. The PANOVA-3 data will be featured in a late-breaking oral presentation on May 31, with Novocure also hosting a dedicated investor event following the session. The company describes TTFields as a multimodal therapeutic platform that may enhance outcomes when combined with chemo, immunotherapy, or radiation.
Nuvation Bio Inc. (NYSE: NUVB) is advancing toward a potential FDA approval for taletrectinib, its next-generation ROS1 inhibitor, with new results also to be showcased at ASCO 2025. The upcoming poster will cover pivotal Phase 2 data from both the China-based TRUST-I and global TRUST-II trials, underscoring efficacy across diverse populations.
"Taletrectinib is a highly selective, next-generation oral tyrosine kinase inhibitor, with the potential to expand what’s possible for patients with ROS1-positive non-small cell lung cancer," said David Hung, M.D., CEO of Nuvation Bio. "We look forward to presenting data from our pivotal TRUST-I and TRUST-II clinical studies, which further demonstrate the potential efficacy and safety of taletrectinib in patients across ethnicities and regions of the world."
Taletrectinib has already been approved in China and granted Breakthrough and Orphan Drug designations by the FDA. A final U.S. approval decision is expected by June 23.
Guardant Health, Inc. (NASDAQ: GH) has expanded its precision oncology platform with a full suite of immunohistochemistry (IHC) tests covering all solid tumors. The launch enhances Guardant’s molecular profiling offering by enabling the detection of key biomarkers like HER2, PD-L1, and c-MET—now a target of newly FDA-approved therapies. This adds to the company’s Guardant360 Tissue test, which integrates genomic and protein data for treatment decision support.
"The information about tumor characteristics garnered from advanced IHC testing will complement the multidimensional insights we are currently providing to oncologists through our recently introduced Guardant360 Tissue multiomic tumor profiling test," said Helmy Eltoukhy, Chairman and Co-CEO of Guardant Health. "This expansion of our portfolio will allow us to offer cancer care teams more comprehensive tissue testing capabilities through a single source to help them make more informed decisions about therapy selection and optimize outcomes for their patients."
SELLAS Life Sciences Group, Inc. (NASDAQ: SLS) has dosed its first pediatric patient in the ongoing Phase 2 trial of SLS009 (tambiciclib) for relapsed/refractory AML, a program supported by Rare Pediatric Disease Designation from the FDA.
“Building upon our promising Cohort 3 data, we are pleased to dose our first pediatric AML patient as part of the ongoing Phase 2 trial,” said Dragan Cicic, M.D., Chief Development Officer of SELLAS. “This milestone reflects our commitment to addressing critical unmet needs in hematologic disorders as we develop treatments for the most difficult to treat patients, particularly pediatric patients, with very few available options.”
The trial is evaluating SLS009 in combination with venetoclax and azacitidine across multiple high-risk AML subtypes, including ASXL1 mutations. If approved, SELLAS may qualify for a Priority Review Voucher—a rare regulatory incentive valued at up to $100 million.
CONTACT:
Baystreet.ca
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.