Survival Data and Regulatory Greenlights Fuel Momentum in Immunotherapy Stocks

Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – Cancer is no longer just a disease of aging populations. Researchers are sounding the alarm over rising rates of early-onset cancer, with a National Cancer Institute (NCI) study in Cancer Discovery highlighting sharp increases in breast, colorectal, kidney, uterine, and pancreatic cases among younger adults. In response, a number of oncology-focused biotechs are advancing promising treatment platforms with upcoming catalysts, including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Plus Therapeutics, Inc. (NASDAQ: PSTV), Tempest Therapeutics, Inc. (NASDAQ: TPST), Intensity Therapeutics, Inc. (NASDAQ: INTS), and AIM ImmunoTech Inc. (NYSE-American: AIM).
The global market for cancer drugs is on track to break the US$900 billion revenue mark by 2034, according to projections from Vision Research Reports. Meanwhile, Precedence Research estimates that next-generation therapies—those leveraging personalized or precision-based approaches—could drive US$175.2 billion in sales over that same horizon, supported by a compound annual growth rate of 7.35%.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) has officially entered the most critical phase of its journey yet - pursuing a potential registration-enabled trial in first-line metastatic pancreatic ductal adenocarcinoma (mPDAC), for its flagship asset, pelareorep. The company announced it has begun formal discussions with the U.S. Food and Drug Administration (FDA) aimed at finalizing a pivotal study design, with trial start-up activities expected to begin before the end of 2025. For investors and potential partners, this marks a clear shift from promising data to regulatory execution in one of the deadliest solid tumors in medicine.
“We expect to move quickly and decisively down a clear regulatory path,” said Jared Kelly, CEO of Oncolytics. “This is about execution and focus. Our goal is to win on survival—and this pivotal study is how we do it. We believe this program not only creates significant value for shareholders but also positions Oncolytics as a highly attractive partner for pharma companies seeking to break open the immunotherapy landscape in mPDAC and other GI tumors.”
The move comes as Oncolytics sharpens its focus on pelareorep, a systemically delivered oncolytic virus designed to convert so-called “cold” tumors—those that are typically invisible to the immune system—into “hot” tumors that can respond to immunotherapy. Pelareorep has been studied across over 1,100 patients and has demonstrated promising results in difficult cancers like mPDAC and HR+/HER2- breast cancer, particularly when used in combination with chemotherapy and immune checkpoint inhibitors.
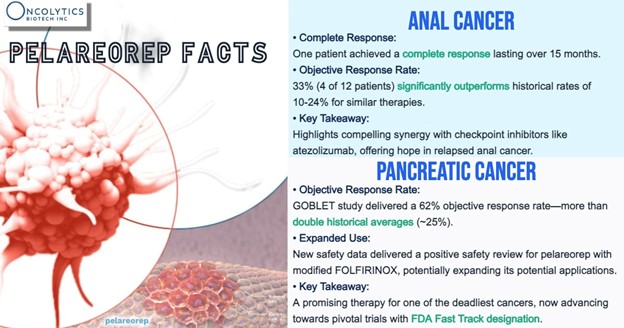
In first-line pancreatic cancer, pelareorep-based regimens have shown a notable 21.9% two-year overall survival rate, compared to a 9.2% historical benchmark. In a separate study pairing pelareorep with chemotherapy and a checkpoint inhibitor, researchers recorded a 62% objective response rate—remarkable given that checkpoint inhibitors are not currently approved for use in this indication.
This robust efficacy signal, along with impressive translational data, helped lay the groundwork for this regulatory step.
“This robust data set, amassed from several studies in cancers that have historically resisted immunotherapeutic approaches, provides definitive validation of pelareorep’s immune-mediated mechanism of action,” said Dr. Thomas Heineman, Chief Medical Officer of Oncolytics. “We observed tumor biopsy-confirmed virus replication, immune cell activation, and the recruitment of cytotoxic T cells into the TME – all consistent with the durable responses observed in patients with metastatic PDAC and HR+/HER2- breast cancer who were treated with pelareorep.”
Earlier this month, Oncolytics hosted a key opinion leader event featuring gastrointestinal cancer experts who reviewed survival outcomes, biomarker validation, and the drug’s potential role in combination therapy. That expert panel helped reinforce the view that pelareorep’s mechanism—activating innate and adaptive immune responses in patients—is both biologically sound and commercially relevant.
Oncolytics’ execution-focused strategy is being led by Jared Kelly and Andrew Aromando, who both played key roles in Ambrx Biopharma’s US$2 billion acquisition by Johnson & Johnson. Kelly was appointed CEO earlier this year, while Aromando recently joined as Chief Business Officer. Together, along with Oncolytics’ already-established team, they’re guiding the company into late-stage development with a sharp focus on capital efficiency and strategic alignment.
Regulatory designations are already in place to accelerate development. Pelareorep holds Fast Track and Orphan Drug designations for pancreatic cancer (and Fast Track for HR+/HER2‑ mBC breast cancer) from the FDA, meaning the agency has already recognized both the drug’s potential and the serious unmet need in this patient population. These statuses not only streamline review processes but also increase the attractiveness of the program to potential pharmaceutical partners.
For context, pancreatic cancer is one of the deadliest common cancers, with a five-year survival rate of less than 14%. Most patients are diagnosed at a metastatic stage, and current treatment options provide limited benefit. Unlike other cancers where immunotherapies like checkpoint inhibitors have transformed care, mPDAC remains largely resistant to those approaches—making the promise of pelareorep especially notable.
What distinguishes pelareorep is its dual capability: it acts as both a viral therapy that replicates in cancer cells and a biological agent that activates the body’s own immune response. Tumor samples have shown increased PD-L1 expression, heightened interferon signaling, and infiltration of tumor-killing T cells—all hallmark indicators that the immune system is being mobilized against cancer. These features are seen as essential for the next generation of immuno-oncology approaches.
With this latest milestone, Oncolytics is entering a phase where the outcomes of FDA interaction will shape not only clinical plans but also potential commercial partnerships. If the agency accepts the company’s proposed trial framework and endpoints—centered around overall survival—the resulting study could become the definitive test for pelareorep’s market potential in mPDAC.
More details on the trial design and timing are expected once FDA feedback is received, but the goal is clear: initiate the pivotal trial start-up activities before the end of 2025 and establish pelareorep as a cornerstone immunotherapy in one of the most underserved cancer settings.
In the meantime, the company remains active in its ongoing breast cancer program and is continuing to explore pelareorep’s potential in other gastrointestinal cancers, including KRAS-mutated colorectal cancer. However, first-line pancreatic cancer has now emerged as the lead strategic focus.
The transition from early-stage promise to registration-stage planning marks a decisive evolution in the Oncolytics story. With data in hand, regulatory engagement underway, and a seasoned leadership team at the helm, Oncolytics is advancing into its most consequential chapter yet.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Plus Therapeutics, Inc. (NASDAQ: PSTV) recently presented[JP2] final results from its ReSPECT-LM trial evaluating REYOBIQ™, a targeted radiotherapeutic, in patients with leptomeningeal metastases (LM). The Phase 1 study showed a promising safety profile and response signal, offering new hope in a setting where survival typically ranges from 2–6 months.
“Previously presented data from ReSPECT-LM was highly encouraging in both the safety profile and response signal in patients with leptomeningeal metastases or LM,” said Michael Rosol, Ph.D., Plus Therapeutics’ Chief Development Officer. “At SNO/ASCO this year, our objective is to present the final clinical trial results and proposed clinical development path forward to clinical leaders in Neuro-oncology and further discuss this in our sponsored symposium.”
The company will also host an expert-led symposium on its LM strategy, reinforcing REYOBIQ’s clinical relevance and platform potential.
Tempest Therapeutics, Inc. (NASDAQ: TPST) received regulatory clearance in China to begin a pivotal trial of amezalpat (TPST-1120) plus standard-of-care in first-line hepatocellular carcinoma. The Phase 3 study builds on prior FDA and EMA support and encouraging early data showing improved survival with the triple combination.
“This regulatory clearance to proceed with a pivotal trial in China is a significant milestone towards reaching global markets where HCC has high prevalence,” said Sam Whiting M.D., Ph.D., chief medical officer and head of R&D of Tempest. “It is rewarding that the regulatory officials in China see the promise and potential of amezalpat and have provided the green light to begin a registration-directed trial. We strongly believe amezalpat is a drug that can make a difference in the lives of patients with HCC and believe it should be advanced to pivotal testing.”
Given China’s high HCC burden, this move expands Tempest’s global reach and potentially accelerates its regulatory timeline.
Intensity Therapeutics, Inc. (NASDAQ: INTS) recently announced that INT230-6 achieved a 100% complete response rate in preclinical models of malignant peripheral nerve sheath tumors (MPNST).
"MPNST is the first neurological-specific tumor for which INT230-6 has been used," said Lewis H. Bender, President and CEO of Intensity. “We are pleased to see that INT230-6 achieved meaningful results in MPMNST that are similar to the results we saw in other in vivo models where INT230-6 has been tested. We look forward to conducting further preclinical research with the Staedtke-Bai laboratory in other neurological cancer models."
The drug, delivered via direct intratumoral injection, combines potent chemotherapeutics with a diffusion enhancer and showed tumor elimination in all treated mice at Johns Hopkins. INT230-6 is also advancing through multiple Phase 2 and Phase 3 clinical trials across solid tumors and sarcomas.
AIM ImmunoTech Inc. (NYSE-American: AIM) recently reported positive mid-year data from its Phase 2 DURIPANC trial of Ampligen® plus AstraZeneca’s Imfinzi® for metastatic pancreatic cancer. Results show encouraging overall survival and progression-free survival trends, with 64% of patients exceeding 6 months OS and a strong safety profile in the post-FOLFIRINOX setting.
“Data from Ampligen as a maintenance monotherapy was extremely positive when compared to existing therapeutic approaches,” said Thomas K. Equels, CEO of AIM ImmunoTech. “DURIPANC builds on that foundation and these results suggest a clear path forward and identify a promising potential benefit of combining the selective innate immune activation of Ampligen with the checkpoint inhibition of durvalumab in pancreatic cancer maintenance therapy.”
The study is a key step toward potential personalized immunotherapy strategies in one of the deadliest cancers.
CONTACT:
Baystreet.ca
(250) 999-4849
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.